Profiles in Leadership / AAI Biosketch
An Interview with Dr. Barry Bloom
by John Emrich and Charles Richter (editors)
October 2020
Presented below are excerpts from one of the AAI Oral History Project’s most recent interviews, with AAI Distinguished Fellow Barry R. Bloom (AAI ’67, president 1985–86). Dr. Bloom is the Joan L. and Julius H. Jacobson Research Professor of Public Health at the Harvard T.H. Chan School of Public Health. Recognized as a leader in global health, Bloom pursues research primarily focused on leprosy and tuberculosis (TB).
This interview was conducted on May 10, 2019, at IMMUNOLOGY2019™ in San Diego. Please note that this interview has been condensed and lightly edited for clarity. To learn more about Dr. Bloom, including his year spent working in the Jimmy Carter White House, click here to view the full interview and transcript.
 Barry R. Bloom Oral History Project interview, 2019
Barry R. Bloom Oral History Project interview, 2019
AAI
Discovering A Passion and Purpose
My background is pretty much undistinguished other than the fact that everybody in my family, all my uncles and one aunt, were physicians, so it was expected from the day I was born that I was going to be a doctor…. In a bolt of adolescent rebellion, I decided not to go to medical school, although I had applied and was admitted, and Rockefeller University had started a new immunology program as a graduate university, and this is what I wanted to do. One of the attractions was…one introductory course, which was one Nobel laureate after another talking about theoretical physics, quantum physics, everything you could think of, much of which we didn’t understand, but we were impressed by the personalities.
Postdoctoral Training Across the Pond
My thesis was published essentially intact in an annual review called Progress in Allergy with, I think, 600 references. Otherwise, I think I would be driving a taxi with no paper and not much to show.
 But because of a connection in the laboratory of a prior immunologist who studied in London, I decided I would study—since I had not gotten very far with cell-mediated immunity—I would study antibodies, and I went to work with professor Rodney R. Porter (AAI ’73) at St. Mary’s Medical School in London. Porter, as you know, won the Nobel Prize for the structure of antibodies, absolutely marvelous guy from Lancashire, very difficult to understand his dialect, but one of the nicest and most generous and intuitively bright individuals I’ve ever met.
But because of a connection in the laboratory of a prior immunologist who studied in London, I decided I would study—since I had not gotten very far with cell-mediated immunity—I would study antibodies, and I went to work with professor Rodney R. Porter (AAI ’73) at St. Mary’s Medical School in London. Porter, as you know, won the Nobel Prize for the structure of antibodies, absolutely marvelous guy from Lancashire, very difficult to understand his dialect, but one of the nicest and most generous and intuitively bright individuals I’ve ever met.
My project was very straightforward. [Porter had] learned that antibodies had two chains. My project was to find out which chain had the active site. I worked really hard, and I worked day and night, and I made my own DEAE [Diethylaminoethyl] columns, because in England at that time, you didn’t buy kits or columns; you made them. So I learned a lot about how to do science, and to make a long story short, I did not find where the active site of antibodies was. [It was] another total failure. It turns out you need both chains to have an active site. I learned a lot about how to separate chains, and I had a lot of negative results.
Embarking on an Academic Career
I had to return and get a job and I didn’t get a huge number of offers, and, for a variety of reasons, wanted to move to New York. My wife was then a student in Asian Studies at Columbia University, so [we] returned to New York, and the original job offer was in the neurology department, because they wouldn’t hire me in microbiology as I didn’t have any papers. But I had an idea of what I wanted to do…. There’s lymphocytes—in those days, we didn’t have T cells—and there were macrophages, and the question that was raging was which cell had specificity for antigen.
My colleague Boyce Bennett spent hours with me working on how to separate cells, and what we showed is that it was lymphocytes, not macrophages, that had the specificity for antigen.
And when we showed that it was the lymphocytes that were inhibiting macrophages, and we showed that as few as a half percent of immune lymphocytes would inhibit the migration of the remainder of normal macrophages, we figured out they must be making something and secreting it…. We called it migration inhibitory factor, and that was really the first of the lymphokines that had been discovered and the first nonantibody product of lymphocytes that had been described in the literature.
So that’s how I got started in the business of cell-mediated immunity, a long series of failures, and for reasons not clear, I somehow lucked out at the end.
Introduction to Global Health
The result of the paper on the lymphokines’ migration inhibitory factor led to a totally unexpected invitation….After we published the Science paper on cytokines and which cell had specificity for antigens, I got an invitation from the World Health Organization (WHO) to come to Geneva to explain my work.
Later, WHO officials arranged a meeting in New Delhi for the following year, and there were three outsiders. One had discovered a colony-stimulating factor, which has had a great power in medicine and in the pharmaceutical industry; one discovered that there was a relationship between sickle cell and malaria; and I was the third of that group. Also there were a series of Indian leprologists, and it was all overseen by a young Norwegian named Tore Godal, who later became head of the Special Programme on Tropical Diseases at WHO and the first director of Gavi [Global Alliance for Vaccines and Immunization]. It was an extraordinary meeting. It was like a clash of two cultures.
Leprosy and TB
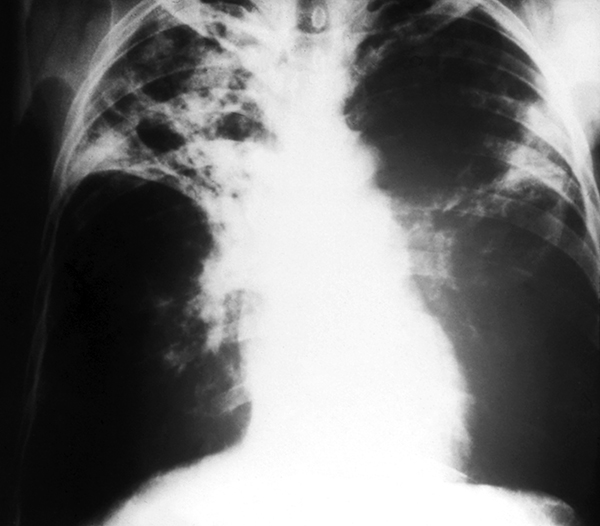 X-ray of lungs with advanced TB
X-ray of lungs with advanced TB
CDCWe knew nothing about leprosy and leprologists knew nothing about basic immunology, and the number of questions we could ask was extraordinary. [And these questions] would be easier to answer in leprosy than almost any other condition, because in contrast to TB, as you know, leprosy is caused by a relative of the tubercle bacillus called Mycobacterium leprae. We can’t study easily what goes on in the human lung. It’s very difficult. But leprosy’s a skin disease. It rarely disseminates internally, probably because it doesn’t grow at high temperatures, and skin is at a lower temperature, about 32 to 34 degrees centigrade.
We knew nothing about leprosy and leprologists knew nothing about basic immunology, and the number of questions we could ask was extraordinary. [And these questions] would be easier to answer in leprosy than almost any other condition, because in contrast to TB, as you know, leprosy is caused by a relative of the tubercle bacillus called Mycobacterium leprae. We can’t study easily what goes on in the human lung. It’s very difficult. But leprosy’s a skin disease. It rarely disseminates internally, probably because it doesn’t grow at high temperatures, and skin is at a lower temperature, about 32 to 34 degrees centigrade.
The second striking thing about this odd disease is that it isn’t a single clinical entity; it’s a spectrum that correlates perfectly with the immunology. At one end of the spectrum, lepromatous leprosy, the bugs flourish. They grow essentially only in macrophages or Schwann cells around the nerves and they cause nerve damage. At the other end of the pole, there’s a massive infiltration of what we would now see as CD4, CD8, and macrophages, and almost no visible bacilli. The macrophages kill off the bacilli, but in the process, they damage the nerves as well. As a consequence, it was an extraordinary, unique opportunity to study the whole range of cellular immune responses from unresponsiveness to too much responsiveness in the context of a human disease. So I have spent a good part of the rest of my life studying immune responses in leprosy, and it has remained, at least for me, a rewarding subject.
 The other extraordinary fascination about leprosy is that there’s no other disease [for which] people in the Middle Ages were buried alive, burned at the stake, or thrown out of cities with a bell and candle and left to survive in the deserts on their own. It not only has a history but it has a stigma, and that led me to believe that, yes, I wanted to do basic science, but not just to write papers in Science, Cell, and Nature; I wanted to do basic science on real diseases with real pathogens, and at the time I started, nobody, or virtually nobody, worked on real antigens.
The other extraordinary fascination about leprosy is that there’s no other disease [for which] people in the Middle Ages were buried alive, burned at the stake, or thrown out of cities with a bell and candle and left to survive in the deserts on their own. It not only has a history but it has a stigma, and that led me to believe that, yes, I wanted to do basic science, but not just to write papers in Science, Cell, and Nature; I wanted to do basic science on real diseases with real pathogens, and at the time I started, nobody, or virtually nobody, worked on real antigens.
This was the era of reductionist science, people working on model systems, so the major antigen was dinitrophenylated bovine serum albumin [DNP-BSA]. [We were] not aware of anybody dying of bovine serum albumin as a major cause of illness, and here we were working on leprosy bacilli in patients and in animal models, particularly interested in the part of the spectrum where the immune response killed off the bugs but caused tissue damage. That struck me as very odd, and if you think of what tuberculosis is like, it’s a massive immune response to wall off the bacilli that causes a hole in the lung and massive tissue damage in the lung. And while we couldn’t get access to lungs, the principles, I thought, were likely to be very similar.
The other possibility was that the tissue damage we saw in skin in leprosy might be relevant to autoimmune diseases, and the particular case of interest was the possibility it might be related to multiple sclerosis, where there was infiltration of white cells into the brain with concomitant damage of nerve cells.
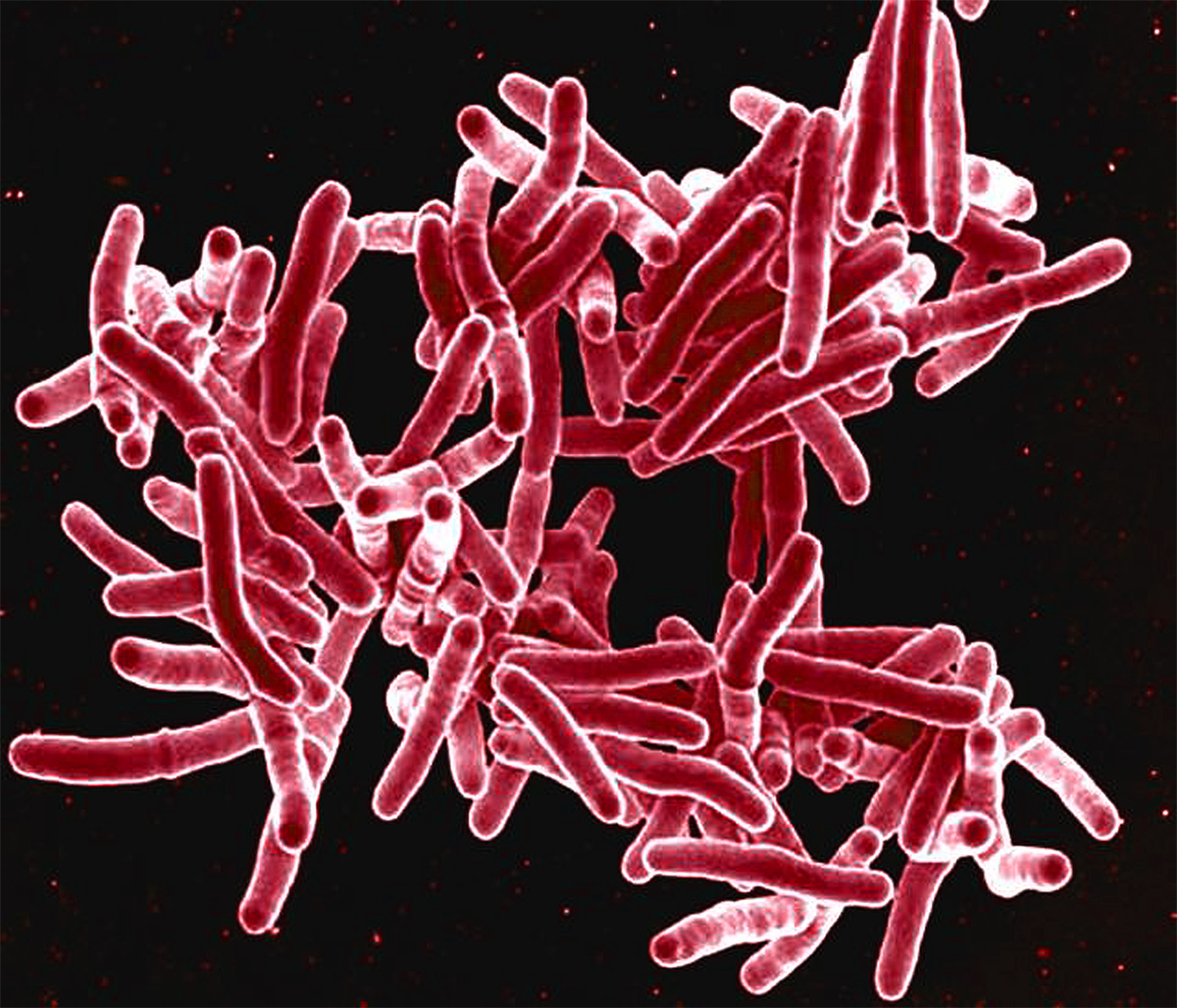 SEM image of Mycobacterium tuberculosis
SEM image of Mycobacterium tuberculosis
CDCSo I flew back from India full of enthusiasm, worked with a terrific neuropathologist at Einstein named Henry Wisniewski, and we did a really simple experiment. We sensitized guinea pigs to tuberculin and then we injected a little tuberculin into the head of some guinea pigs to see what would happen, and what would happen was astonishing: a massive cellular response, a dissociation of the sutures that hold the brain together, and the guinea pigs’ heads blew up. So we had created an artificial neurologic autoimmune disease by creating a specific immune response to a foreign antigen in the vicinity of nerve cells in the brain, and the specific response to the tuberculin led to a nonspecific damage of the nerve cells. That’s what goes on in tuberculoid leprosy, and, to some extent, that may also go on in some autoimmune diseases elsewhere in the body, and that was called “bystander demyelination.”
Collaborations
From there, I’ve worked for a large part of my career in collaboration with wonderful students and fellows. The first that I would mention on this occasion is one of my early postdocs, JoAnne Flynn (AAI ’96, president 2018–19), who came with a background in microbiology and is now [2019], I’m proud to say, president of the American Association of Immunologists.
As JoAnne came to the lab, she was really good at microbiology and genetics and really didn’t know anything much about immunology, so we gave her some immunology projects that turned out to be absolutely transformative for the field. It was at that time that knockout mice became available, and so we got mice that had knockouts in gamma interferon, and they died from TB very rapidly, at 21 days. We had mice that lacked tumor necrosis factor [(TNF)] and we assumed those mice would not show pathology, but, in fact, they died at the same time as the gamma interferon knockouts. We reconstructed the mice to show that if you triggered both, you need both interferon gamma and TNF to get a protective response in the mice.
JoAnne then asked, what about killer cells? And she used mice with knocked out MHC Class 1 and showed that CD8 and cytotoxic cells seemed to be important for protection. JoAnne really laid the basis for the fundamental cellular mechanisms of protection, in cellular terms, of how you get protection against leprosy and how you get protection against TB. We still don’t know in molecular terms, any more than I did when I was a graduate student, about what molecules are really crucial for assuring protection and being necessary to develop rationally a perfect vaccine.
But to continue my studies in leprosy, [Robert Modlin (AAI ’86) and I] found that there was a mechanism in humans, at least for killing TB in vitro, that depending on products of activated lymphocytes, probably interferon gamma, and other cytokines, and it worked by a mechanism totally different than what we found in mice. So in my lab, John Chan (AAI ’06) and some other students and postdocs showed the major mechanism for killing TB in mice was not oxygen radicals, which is what killed most other bugs at the time, in terms of our knowledge, but it was killed in mice by reactive nitrogen oxides and reactive nitrogen species.
 Nine-banded armadillo
Nine-banded armadillo
CDCWe showed that human macrophage is killed by a different mechanism, in vitro at least, and the mechanism required the engagement of vitamin D. We worked out a completely unique pathway where vitamin D led to the production not of radicals but of a protease or an antimicrobial compound that had the ability to put a hole in the membrane of the very tough membranes of Mycobacterium tuberculosis and leprae.
To make a very long story short, we’ve worked out a lot of the mechanisms important for activating macrophages, at least in vitro, to control and kill TB, and we also showed that one of the things that the lymphocytes of the old days—now, CD8 T cells—did is a subset of them could not only kill infected macrophages, they could kill the TB within the macrophages. With the help of a young junior faculty named Sam Balin and Robert Modlin’s lab at UCLA, we showed that this requires a subset of killer cells that is able to put a hole in the membrane of the target cell with perforin and deliver antimicrobicidal compounds granzyme and granulysin.
The Importance of the WHO
When I was invited in 1968, I think, to go to India for the first time, it’s an experience I really never got over, and I was absolutely motivated to try to use science in some way, not to make drugs and vaccines in my lab, but [to] understand the basic science that might allow others to do that [and] target it on diseases of the Third World. As a result of that, this wonderful Norwegian who had created the first leprosy center in Africa....was invited to return to WHO and set up a program on leprosy, and he asked me then to join him as an advisor to that program that was called IMMLEP [Steering Committee on the Immunology of Leprosy]. It was funded, tiny amounts of money, by the Norwegian government, and my role was to try to get some of the best scientists in the world to come for nothing to WHO and share their ideas and share something else as well.
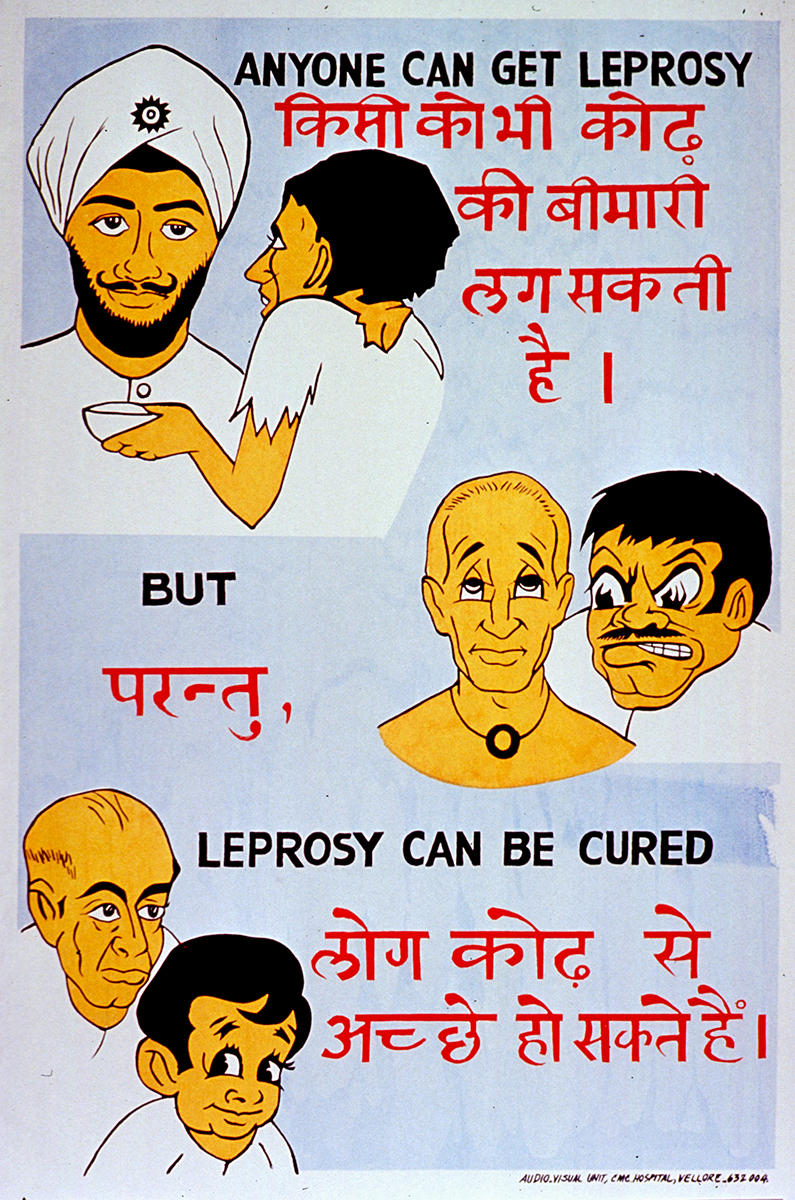 Leprosy public health poster, India
Leprosy public health poster, India
National Library of MedicineIt turns out the leprosy bacillus was discovered seven years before Robert Koch discovered the tubercle bacillus. It has never been able to be grown in a test tube. It is a completely genetically degenerate organism. It barely can survive in vivo. It’s amazing that it actually causes a disease. Nonetheless, how do you study leprosy if you can’t study the bug? It doesn’t grow. Except there was a wonderful guy at CDC [Centers for Disease Control and Prevention] named Charles C. Shepard (AAI ’51) with another group who showed that it did grow in two animals.
It grew in the footpad of mice, which has low body temperature, but you can’t get a lot of bacilli out of a footpad in a mouse to study the bug worldwide. It also grew in a weird animal called the nine-banded armadillo, Dasypus novemcinctus, and these are all over the southeast of the United States. They have really crappy immune systems. Because they’re encoated with an armor coating, they don’t need much of an immune system, and I believe that that’s the major reason M. leprae grows. They have a low body temperature and they have a lousy immune system.
A contract was let by WHO and enabled two major laboratories studying leprosy to grow enough bacilli in armadillo livers—you could get 1010 per gram of tissue. That’s a lot of bacilli. WHO organized that, the IMMLEP Committee oversaw that, and the bacilli that were obtained were made available at no cost to any scientist in the world qualified to study leprosy. While with many other infectious diseases, we’re worried about giving anything away because they were interested in setting up companies, we were pretty sure there was not going to be a lot of money to be made from a company that worked on leprosy. My lab and another lab elsewhere simultaneously made the first monoclonal antibodies against antigens of M. leprae and M. tuberculosis. We gave that to WHO, and that was distributed free of charge to everybody that wanted it in the world.
Then I was privileged to meet at WHO with two giants in the field of molecular genetics, Ron [Ronald W.] Davis at Stanford and Rick [Richard A.] Young at MIT, who had invented the first really useful gene expression system, where you could clone genes into a phage lambda gt10 or gt11 and make foreign proteins from almost anything in E. coli, and thus we had the ability to manufacture or at least allow laboratories to make buckets of any TB or M. leprae antigen. And in the course of it, the DNA enabled the sequence of M. leprae and M. tuberculosis to be done. None of this would have gotten done had not a wonderful collection of people outside the field of immunology, outside the field of genetics, been willing to work for WHO for a common purpose to use their skills and knowledge and to make everything that we discovered free and open to anybody in the world.
 So I became heavily involved then in WHO activities. I was the first chair of the outside advisory committee called STAC, Scientific and Technical Advisory Committee, to the leprosy program....Since it was such a model program that did the science and gave it all away, that led to the origins of the so-called Tropical Disease Research Programme [ed. Special Programme for Research and Training in Tropical Diseases (TDR)], which WHO then created for diseases like malaria, leishmaniasis, filariasis, and the hope was that they would also pull scientists from all sorts of fields together to move forward on these now-called neglected tropical diseases. So that has been an extraordinary and wonderful experience.
So I became heavily involved then in WHO activities. I was the first chair of the outside advisory committee called STAC, Scientific and Technical Advisory Committee, to the leprosy program....Since it was such a model program that did the science and gave it all away, that led to the origins of the so-called Tropical Disease Research Programme [ed. Special Programme for Research and Training in Tropical Diseases (TDR)], which WHO then created for diseases like malaria, leishmaniasis, filariasis, and the hope was that they would also pull scientists from all sorts of fields together to move forward on these now-called neglected tropical diseases. So that has been an extraordinary and wonderful experience.
Then I switched. They created a vaccine program and supported research, and I chaired the committee called IMMTUB, the Immunology of Tuberculosis, and that has spurred on efforts to develop the basic science underlying vaccines. In the last year, we’ve seen two papers in The New England Journal [of Medicine] that indicate there are now hopes for having better ways to immunize people than we’ve had for the last 100 years. So immunology is really making an impact on two almost totally refractory diseases. TB is now the largest cause of death in the world from any infectious disease, exceeding HIV and malaria for the first time, so this is a serious effort that WHO has inspired and now many labs are contributing to.
People forget that essentially all vaccines are iterative processes. The first go is never the perfect vaccine, and there is a history of almost all vaccines requiring continuous improvement. So I’m not confident that these two papers are the last word, but if they show in larger studies the kind of protection, 50 percent, or for people under 25 years of age—one of them showed 84-percent protection—when you’re getting 10.7 million new cases a year, I’ll take a 50-percent effective vaccine any day. It would be a huge impact.
Leprosy Today
So the status of leprosy is that there was a counterpart committee to IMMLEP, which was called TLEP, the mission of which was to develop drugs, and they developed drugs, tested them in mouse models. The situation with leprosy is both interesting and somewhat discouraging. It is interesting because that treatment has been applied to those countries that have a leprosy problem. When I started, there were 12.5 million registered—leprosy is a notifiable disease to all governments. There were 12.5 million patients registered to have leprosy. Assuming that was 50 percent of all the patients, that half were missed, not least because of the stigma and their reluctance to come on, there must have been 25 million at the start of those IMMLEP and TLEP programs. There are now fewer than 800,000 registered new patients, an astonishingly effective impact of drugs, mostly on people who already had the disease. It’s a very, very slow disease.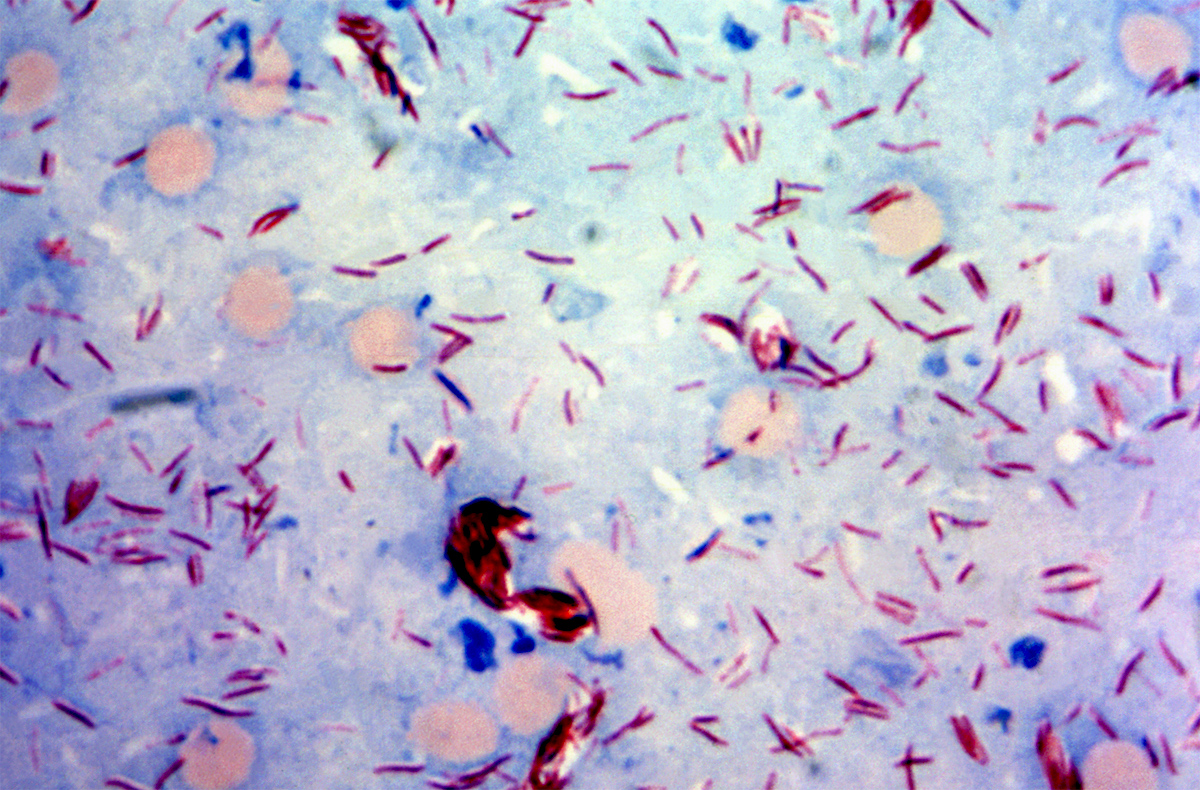 Mycobacterium leprae
Mycobacterium leprae
CDC
The discouraging part is the incidence rates. The number of new cases has not fallen, and what that means is people are transmitting leprosy before they know they have it and go in for treatment. If they do go in for treatment, they get cured, 90-some percent cures, not a problem.
What’s really important there is the only way we have to test a vaccine is very expensive long-term trials in patients at risk for TB, which is a low percentage, even in high-burden areas. What we really need to know is what are the immunologic correlates that guarantee protection. We know for polio if you have neutralizing antibody, you’re protected. Done. For hepatitis B, it’s exactly the same, and we even know what isotype of antibody guarantees protection. We haven’t got a clue what are the essential ingredients to guarantee if you saw these cytokines, these T cells, these NK cells, and innate immune cells, this vaccine worked, this patient is protected. So we have lots of immunology yet to do.
Science and the Public
I look at my role as, in many of these aspects, [one] of making mischief, and so the current cause of mischief with which I am engaged has to do with the issue of outbreaks of vaccinepreventable diseases in the U.S. As an immunologist, you can’t ignore the fact that we have now more measles cases than we’ve had in the last 20 years. We have a great vaccine for measles, mumps, and German measles. How is that possible in America? What does it mean to have religious and moral objections to a bloody vaccine that saves lives, and what are we going to do about it?
 So in this effort, I can just say that in two editorials, one in The Washington Post recently and one in The Journal of the American Medical Association hooking up with a major educator, Scott Ratzan, and Larry Gostin, who’s head of Health Law at Georgetown, we’ve put together a set of proposals of how we can tighten up the immunization in this country particularly, to cut out the efforts, quite successful at this point, of the anti-vaccine groups by trying to get the truth about safety and efficacy of vaccines and protect our kids who don’t get a vote on this. Kids don’t vote on whether they’re going to be protected against measles or blindness or deafness or encephalitis or pneumonia; their parents do. And as a result, we’ve got to get more persuasive in protecting those kids with the tools we have.
So in this effort, I can just say that in two editorials, one in The Washington Post recently and one in The Journal of the American Medical Association hooking up with a major educator, Scott Ratzan, and Larry Gostin, who’s head of Health Law at Georgetown, we’ve put together a set of proposals of how we can tighten up the immunization in this country particularly, to cut out the efforts, quite successful at this point, of the anti-vaccine groups by trying to get the truth about safety and efficacy of vaccines and protect our kids who don’t get a vote on this. Kids don’t vote on whether they’re going to be protected against measles or blindness or deafness or encephalitis or pneumonia; their parents do. And as a result, we’ve got to get more persuasive in protecting those kids with the tools we have.
So that’s the effort that I’m engaged in, and have been quite pleased in the last couple weeks. A whole slew of vaccine experts, which I don’t pretend to be, and health law experts and people administrating health programs in universities have started to join and say, “We have to be able to do something to increase the level of awareness and understanding, deal with the social media, with misinformation. Vaccines don’t cause autism. Vaccines do save lives.” That seems to be a battle to take on the politicians right now in—there are only two states that have not had [measles] outbreaks, and they’re the states you wouldn’t guess—Mississippi and West Virginia—who do not have exemptions, other than medical exemptions, for vaccines, and they have had no recent outbreaks. So it’s telling you something, and we have to begin to deal with that at a legislative level in states to protect our kids.
The Diverse and Exciting Field of Immunology
Where can you get a field where you can study fundamental immune responses, regulation of a really complicated system? The dean at Albert Einstein when I was there was a neuroanatomist, and he liked to say to students, who always roared at this, that the brain is the second-most important organ of the body, and he never specified what the other organ was. For me, it was always the immune system and lymphoid system, even more complicated, probably, than the brain. The ability to recognize almost any compound, any chemical in the environment that it’s never seen before and make a response to it, that’s astonishing. And then to be able to go from very basic mechanisms, from DNA and RNA and all that, to how do you develop a vaccine or a diagnostic that’s going to make a difference in the world, I mean, [in] how many fields can one investigator have the freedom to find their niche in any part of a giant scientific spectrum? So it remains even more exciting now than it was when I was a student.
References
- Bloom, Barry R. Bloom and Boyce Bennett. “Mechanism of a reaction in vitro associated with delayed type hypersensitivity.” Science 153, no. 3731.