Polio: Part III—The Vaccine
by John S. Emrich and Charles Richter
July 2021, page 24–29
Once John F. Enders (AAI 1936, AAI president from 1952–53), Thomas Weller (AAI 1952), and Frederick Robbins (AAI 1952) had successfully cultured the poliovirus, the possibility of an effective vaccine for the worsening scourge of polio was in sight. The basic science breakthrough for replicating poliovirus occurred in 1949, a year also marked by a huge surge in polio cases. Polio case rates had been increasing since 1942, but 1948 was the first year to exceed the rates of the initial major epidemic in 1916, with 28.07 cases per 100,000 Americans.
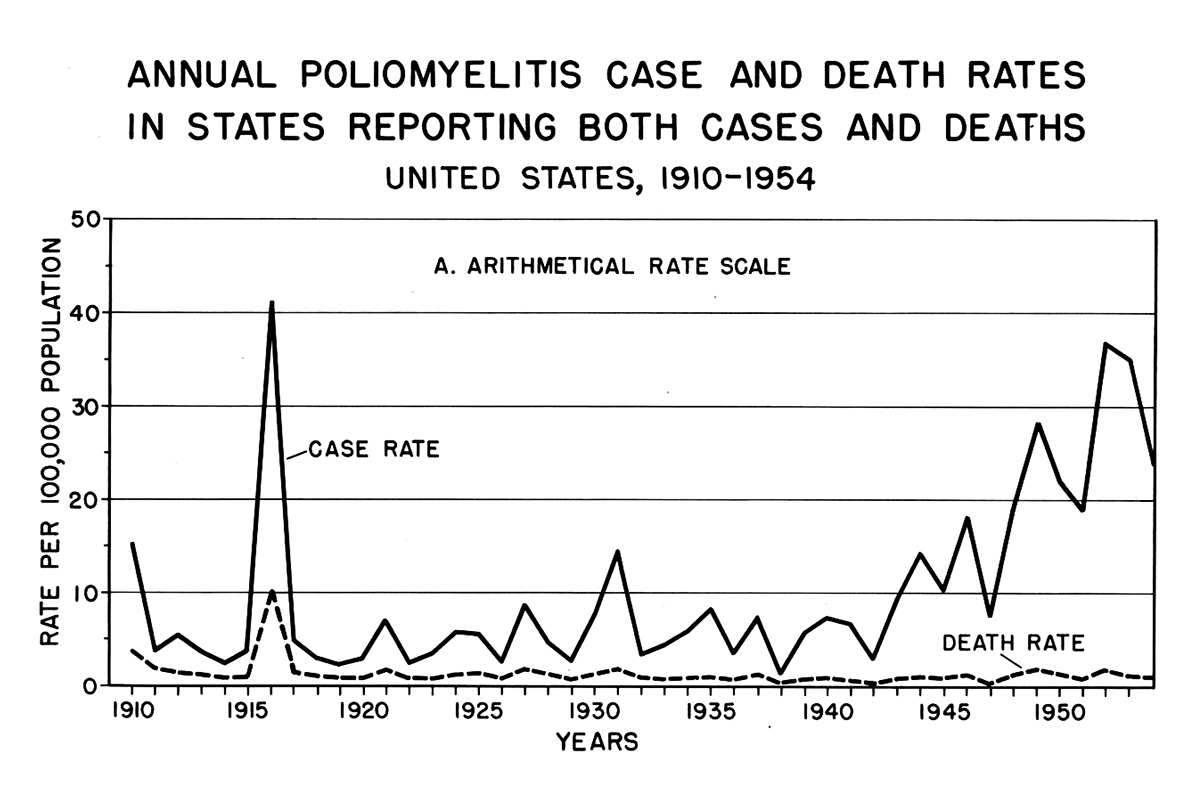 Polio cases in the United States, 1910–54
Polio cases in the United States, 1910–54
Eisenhower Presidential LibraryThe rate increase was a reflection of the rebounding birthrate from its nadir during the Great Depression, together with the massive expansion of military service personnel after the United States entered the Second World War in December 1941. The birthrate slowly increased from a low in 1936 through 1944; a slight lull in 1945 was followed by a “baby boom” beginning in 1946. Because polio was primarily a disease of the young, this increasing population of children in the United States provided the virus with more potential victims and carriers.
The need for a vaccine was as pressing as it had been during the 1916 outbreak, except this time there was a light at the end of the tunnel. Multiple researchers were actively developing new vaccines based on a clearer understanding of poliovirus.
Hilary Koprowski
On the Pearl River, New York, campus of American Cyanamid Company in 1947, Hilary Koprowski (AAI 1946) began working independently on a polio vaccine unbeknownst to his superiors. Having previously worked with Max Theiler on his successful yellow fever vaccine, Koprowski believed he could use the same attenuation process with poliovirus. His attenuated live strain oral vaccine proved successful in preliminary animal trials, so Koprowski administered the oral vaccine to himself in 1948—and it resulted in an antibody response against the poliovirus.
 Postcard of Lederle Laboratories, c. 1955
Postcard of Lederle Laboratories, c. 1955
New York HeritageIn 1950, Koprowski conducted his first clinical trial on 20 disabled children in Rockland County, New York. The trial was a success as all the children showed a positive antibody response, excreted the attenuated strain, and, in all but two cases, were not susceptible to reinfection via the attenuated virus. When the initial results became public, Koprowski was met with swift national and international condemnation about his choice of subjects (clinical consent laws were far more permissive at that time). Despite the pushback, Koprowski and American Cyanamid continued with their polio vaccine research.
Jonas Salk
Jonas Salk (AAI 1947) was born in New York City to poor Ashkenazi Jewish parents; his father was a first-generation American born in New Jersey, and his mother fled Russia to the United States when she was 12. Salk was nearly two years old when the 1916 polio epidemic gripped the city. Intermittent polio outbreaks continued in New York as Salk grew up. He completed his primary education, graduated from the City College of New York, and enrolled at New York University (NYU) College of Medicine in 1934 with the intention of becoming a researcher rather than a clinician. That same year, Thomas Francis, Jr. (AAI 1930, AAI president 1949–50) isolated the influenza A virus at the Rockefeller Institute for Medical Research.
Salk became especially interested in bacteriology and problems of immunization, and toward the end of his medical training, he benefited greatly from the mentorship of Francis, who had moved to NYU College of Medicine. In 1940, Francis isolated the influenza B virus while Salk was on the staff of Mount Sinai Hospital. As Salk later described it, he “saw the opportunity…to test the question as to whether we could destroy the virus infectivity and still immunize.”
In 1942, Salk followed Francis to the University of Michigan School of Public Health, where they created a killed-virus vaccine for influenza. Three years later, the United States Army—which had been the major funder of the research—administered the vaccine to eight million soldiers, reducing their rate of infection by 92 percent in that year’s epidemic. This success would be the model for Salk’s later work on polio.
The Salk Vaccine
In 1947, seeking to gain some independence from his mentor, Salk took a position at the University of Pittsburgh School of Medicine, where he initially continued work on influenza. Shortly after he arrived, the director of the National Foundation for Infantile Paralysis (NFIP) asked him to participate in a poliovirus typing program. That research was not especially exciting to Salk. He later said that most people looked on it as “routine drudgery,” but it gave him a comprehensive and fundamental understanding of polio at just the right moment as Enders, Weller, and Robbins were successfully cultivating the virus.
 Child watches Jonas Salk administer polio shot on TV, 1955
Child watches Jonas Salk administer polio shot on TV, 1955
National Library of MedicineEnders and his colleagues were at odds about whether to steer their research towards creating a vaccine. Enders argued against it because he did not view vaccines as basic research, and their laboratory was ill-suited to transition to polio vaccine research. His argument prevailed and samples, data, and techniques were shared with Salk and his laboratory in Pittsburgh.
Salk, with NFIP funding, built on the work of Enders, Weller, and Robbins, and applied the techniques he had refined with influenza to develop a killed-virus vaccine for polio. The driving principle behind using inactivated virus was to streamline the testing process to get a vaccine to the public as quickly as possible.
After growing large quantities of poliovirus in a culture of monkey kidney cells, Salk, along with his small research team that included Julius Youngner (AAI 1950), Byron Bennett, and L. James Lewis, killed the virus with formaldehyde and injected this inactivated polio vaccine (IPV) into monkeys. When the tests showed that the vaccine produced immunity as evidenced by a specific antibody response, Salk moved on to humans. The clinical trials of the Salk vaccine began in 1952. All trials were overseen by Thomas M. Rivers (AAI 1921, AAI president 1933–34), a renowned virologist who was chair of the NFIP committees on research and vaccine advisory.
 Line for polio vaccine, Texas
Line for polio vaccine, Texas
CDCThe first small trials were conducted at institutions near Pittsburgh—the D.T. Watson Home for Crippled Children and the Polk State School, established for “feeble-minded” children of western Pennsylvania—and successfully demonstrated antibody production in humans after vaccination. The following year, a pilot study with 15,000 children (including Salk’s own sons) was undertaken to optimize the vaccine schedule.
Polio Pioneers
In 1954, the largest field trial of a vaccine in history began. Designed and led by Francis, now the director of the University of Michigan Poliomyelitis Vaccine Evaluation Center, the year-long nationwide clinical trial was conducted by over 100 researchers on nearly two million children who volunteered for the study—some receiving the vaccine, and others a placebo—at a cost of over $17 million (more than $169 million in 2021 dollars).
All the volunteers received a “Polio Pioneer” card certifying their participation. On April 12, 1955, the 10-year anniversary of President Franklin Roosevelt’s death, a national and international press contingent arrived at the overfilled Rackham Auditorium of the University of Michigan, where Francis declared on live television that the Salk vaccine was safe and effective. Later that day in an interview with Edward R. Murrow, Salk told the celebrated interviewer that “there is no patent. Could you patent the sun?”
With the vaccine approved, the federal government wanted a quick rollout to prevent another epidemic as summer approached. Within hours of the vaccine’s approval, the NIH Laboratory of Biologics Control (LBC), under the authority of Secretary of Health, Education and Welfare Oveta Culp Hobby, licensed six companies to produce it: Eli Lilly, Parke-Davis, Wyeth, Sharp & Dohme, Pitman-Moore, and Cutter Laboratories.
 Cart load of Wyeth vaccine, 1963
Cart load of Wyeth vaccine, 1963
CDCAfter two weeks, these companies had produced only 10 million doses, nearly all of which had been promised to the NFIP at no charge for an initial round of immunizations of first- and second-graders as well as the “Polio Pioneers” who had received the placebo, leaving the vast majority of children unprotected. The executive branch had no plan for distribution, reflecting the assumption that it was best left up to the free market. After a massive public outcry, President Dwight Eisenhower committed to “a large federal role in the distribution and financing of this vaccine.”
Throughout the spring and summer of 1955, children around the country lined up to receive their shot at local elementary schools, a logistical marvel that involved training 60,000 medical personnel, 64,000 teachers and principals, and 220,000 volunteers.
The Cutter Incident
Only two weeks into the initial vaccine rollout, reports of polio symptoms in a few vaccine recipients began to emerge. The surgeon general placed a pause on all vaccinations on May 8, 1955, while the cause was determined. Investigators discovered that Cutter Laboratories had released a batch of 120,000 doses of the IPV that contained live poliovirus.
The error’s cause was cell debris that prevented sufficient exposure of the virus to the inactivating agent. Additionally, poor oversight from the LBC allowed the active-virus doses to be distributed to and injected at vaccination sites. A third of these doses resulted in children contracting abortive poliomyelitis, a form which produces minor symptoms, because it does not involve the central nervous system, but which is still transmissible. Worse, 56 children developed the paralytic form of the disease, resulting in five deaths. Another five children died and 113 were paralyzed after contracting polio from one of the vaccine recipients.
The Cutter incident was one of the worst pharmaceutical disasters of all time, but the comprehensive investigative response and increased federal safety protocols it triggered ensured that the 400 million doses of the Salk vaccine produced from 1955 to 1962 were safe and effective. Several of the officials involved in the original licensing and safety decisions resigned, including Secretary Hobby and NIH Director William H. Sebrell Jr.
The children and families who benefitted from successful vaccination responded with a flood of letters expressing their appreciation and relief at being freed from the horrible dread of polio. Looking back on the tragedy, John Enders wrote that the lesson to be learned was that “we must never again allow decisions about essentially scientific matters to be made for us by people without training or insight.”
Albert Sabin
 Albert Sabin, 1957
Albert Sabin, 1957
National Library of MedicineAlbert Sabin (AAI 1946) was born in Białystok, Poland, in 1906. His family immigrated to the United States in 1921 to escape growing violent antisemitism and settled in Patterson, NJ, in the shadow of New York City. In 1923 Sabin enrolled at NYU, earning his bachelor’s degree in 1928 and a medical degree in 1931.
His research toward a polio vaccine began years before Salk’s. In 1936, working with Peter Olitsky (AAI 1917) at the Rockefeller Institute, Sabin had been successful in cultivating poliovirus in vitro in human embryonic nervous tissue, but that experiment had used a strain that had undergone 20 years of brain-to-brain passage in experimental monkeys. Thus, when the same virus failed to grow in non-nervous tissue, it appeared to confirm the common finding that polio was only a disease of the nervous system.
Five years later, however, Sabin, now at Cincinnati Children’s Hospital in Ohio, demonstrated that the presence of the poliovirus in the alimentary canals of deceased humans indicated that this canal was in fact the “primary localization or portal of entry.” Instead of relying on samples sent to his laboratory, Sabin and his assistants personally travelled to morgues in Ohio, Indiana, and West Virginia during an epidemic in 1940 to perform autopsies on every polio fatality they could. Their extreme precautions regarding sterile instruments allowed them to confirm that the poliovirus entered the body via the gastrointestinal tract rather than the nasal route.
As occurred with so many other scientists at the time, Sabin’s work on polio was interrupted by the Second World War. He remained focused on virus research, however, developing effective vaccines for three mosquito-borne flavivirus diseases: St. Louis encephalitis, Japanese B encephalitis, and dengue. Much of this research was published in The Journal of Immunology (The JI).
When Enders, Weller, and Robbins developed the technique to culture the poliovirus, Sabin began work on a live-virus vaccine, attenuated by being passed though monkey tissue repeatedly. This oral polio vaccine (OPV) produced immunity faster than the IPV, provided both humoral and cell-mediated immunity, and entered the body by the digestive system just like the actual virus.
Sabin’s first trial, in the winter of 1954–55, was on 30 prisoners at the United States Industrial Reformatory in Chilicothe, Ohio, a federal prison that had held Charles Manson as a young adult. The next step was a large-scale field trial, but after the Cutter incident, it was unlikely that Sabin would be able to arrange one in the United States.
OPV Trials Abroad
 Preparing doses of OPV
Preparing doses of OPV
CDCBy the mid-1950s, Sabin was not the only one with a new polio vaccine ready for foreign trials. Koprowski, following small clinical trials in the United States, was also searching for a country where he could test his vaccine. In 1956 Ireland agreed to a small clinical trial of children with parental consent in Belfast. Initially the trial went well. The children had no ill effect from the oral vaccine and exhibited a positive antibody response. Unfortunately, some fecal samples from the children showed that the virus regained some potency after it passed through the digestive tract of the trial subjects.
Two years later, Sabin was able to convince the Soviet Union to allow a field trial of his live-virus vaccine using five times the number of children involved in the Salk trial: 10 million participants, all of whom received the vaccine, with no control group. The USSR had experienced its first widespread epidemics of polio only after the Second World War, but since then had suffered major outbreaks in all of its republics. The OPV, given in a sugar cube, was easier to administer than an injection, and compared to the Koprowski vaccine, had the benefit that recipients would shed weakened vaccine—rather than potent virus—in their stool.
Research at Annual Meeting and in The JI
At the post-war AAI annual meetings, polio was an increasingly frequent topic of discussion. Two talks at the 1947 meeting, the first AAI meeting held after wartime restrictions were lifted, focused on the recovery of the virus and distribution of antibodies in paralyzed monkeys. The 1949 meeting included three talks addressing comparative studies of the different strains of poliovirus. By 1951 the seven polio talks benefited from the culturing breakthrough, covering a variety of aspects of cultivation as well as attempts to produce immunity. Speakers who presented their research at these meetings included Salk, Sabin, Francis, Youngner, Joseph L. Melnick (AAI 1948), and Robert Ward (AAI 1951).
 CDC polio vaccine poster, 1963
CDC polio vaccine poster, 1963
CDCDuring the same period, The JI continued to publish many articles on new developments made possible now that the poliovirus could be readily cultured. After the publication of the Enders, Weller, and Robbins breakthrough, 65 articles on polio in The JI came from research funded by the NFIP—about twice as many in nine years as in the previous 17.
Legacy of Polio Vaccines
In 1961, the Sabin OPV was approved in the United States. It overtook the Salk IPV due to its ease to administer and the more robust and longer lasting immunity it provided. These advantages were especially pronounced in countries where polio was endemic: the need for sterile syringes made the IPV unsuitable for mass vaccinations, and the OPV provided immunity in the intestinal tract, which aided in preventing infection by the wild-type strain of the poliovirus.
In 1994, polio was declared eradicated from the Americas thanks to the two vaccines. With the threat of polio almost non-existent, the U.S. government recommended a return to IPV in 1999 to avoid any chance of a recipient contracting polio from the attenuated virus in the Sabin vaccine. Since then, the Salk vaccine has remained the standard in the United States. Today, the World Health Organization recommends a combination of the OPV and IPV in areas where polio is endemic or new outbreaks occur.
During the worst polio epidemics of the 1950s, parents were terrified that their children would catch the disease and suffer lasting pain and paralysis or death. They leapt at the chance to immunize their children even as part of a field trial. Today, as the world begins to benefit from rapidly developed COVID-19 vaccines, many people remain skeptical about receiving them, spurning even those that use the new mRNA technology to produce immunity without exposing the recipient either to killed or attenuated virus.
See also articles that were part of this series:
Notes:
The following are all published in The Journal of Immunology:
Albert B. Sabin and Alex J. Steigman, “Studies on Local Antibody Formation in the Nervous System of Paralyzed, Poliomyelitis Convalescent Monkeys,” 63, no. 2: 211–8; Robert M. Chanock and Albert B. Sabin, “The Hemagglutinin of St. Louis Encephalitis Virus: I. Recovery of Stable Hemagglutinin from the Brains of Infected Mice,” 70, no. 3: 271–85; “The Hemagglutinin of St. Louis Encephalitis Virus: II. Physico-Chemical Properties and Nature of Its Reaction with Erythrocytes,” 70, no. 3: 286–301; “The Hemagglutinin of St. Louis Encephalitis Virus: III. Properties of Normal Inhibitors and Specific Antibody; Use of Hemagglutination-Inhibition for Diagnosis of Infection,” 70, no. 3: 271–85; “The Hemagglutinin of Western Equine Encephalitis Virus: Recovery, Properties and Use for Diagnosis,” 73, no. 5: 337–51; “The Hemagglutinin of West Nile Virus: Recovery, Properties, and Antigenic Relationships,” 73, no. 5: 352–62; Benjamin H. Sweet and Albert B. Sabin, “Properties and Antigenic Relationships of Hemagglutinins Associated with the Dengue Viruses,” 73, no. 5: 363–73.
References
- Guinn, Jeff. Manson: The Life and Times of Charles Manson. New York: Simon & Schuster, 2013.
- Hostrman, Dorothy M. “The Sabin Live Polio Vaccination Trials in the USSR, 1959,” Yale Journal of Biology and Medicine 64.
- Jacobs, Charlotte. Jonas Salk: A Life. New York: Oxford, 2015.
- Juskewitch, Justin E., Carmen J. Tapia, and Anthony J. Windebank. “Lessons from the Salk Polio Vaccine: Methods for and Risks of Rapid Translation.” Clinical and Translational Science 3, no. 4.
- Koprowski, Hilary, Thomas Norton, Klaus Hummeler, et al. “Immunization of Infants with Living Attenuated Poliomyelitis Virus: Laboratory Investigations of Alimentary Infection and Antibody Response in Infants Under Six Months of Age with Congenitally Acquired Antibodies.” Journal of the American Medical Association 162, no. 14.
- Melnick, Joseph L. “My Role in the Discovery and Classification of the Enteroviruses.” Annual Review of Microbiology 50.
- Offit, Paul. The Cutter Incident. New Haven: Yale, 2005.
- Offit, Paul. “The Cutter Incident, 50 Years Later.” New England Journal of Medicine 352, no. 14.
- Oshinsky, David M. Polio: An American Story. New York: Oxford, 2005.
- Roca-Garcia, Manuel, Hilary Koprowski, G.A. Jervis, et al. “Immunization of Humans with A Chick Embryo Adapted Strain of Mef1 Poliomyelitis Virus.” The Journal of Immunology 77, no. 2.
- Sabin, Albert and Peter Olitsky. “Cultivation of Poliomyelitis Virus in vitro in Human Embryonic Nervous Tissue.” Proceedings of the Society for Experimental Biology and Medicine 34 no. 3.
- Williams, Gareth. Paralysed With Fear: The Story of Polio. New York: Palgrave Macmillian, 2015.
- Jonas Salk Interview: Congressional Gold Medal. Academy of Achievement. https://achievement.org/achiever/jonas-salk-m-d/#interview (accessed April 19, 2021).
- “Polio vaccine comes full circle; Controversy: After years of debate, experts choose safer injections over oral vaccines.” Baltimore Sun. 16 March 1999.
- “Reported paralytic polio cases and deaths in the United States since 1910.” Our World in Data. https://ourworldindata.org/grapher/reported-paralytic-polio-cases-and-deaths-in-the-united-states-since-1910?country=~USA (accessed April 19, 2021).
- “Research Starters: US Military by the Numbers.” The National WWII Museum. https://www.nationalww2museum.org/students-teachers/student-resources/research-starters/research-starters-us-military-numbers (accessed April 27, 2021).
- “Soviet Trials of Sabin’s Live Poliovirus Vaccine.” History of Vaccines.https://www.historyofvaccines.org/timeline#EVT_100330 (accessed April 19, 2021).
- “Table: 1-1. Live births, birth rates, and fertility rates, by race: United States, 1909–2003.” of the Vital Statistics of the United States, 2003, Volume I, Natality. https://www.cdc.gov/nchs/data/statab/natfinal2003.annvol1_01.pdf (accessed April 27, 2021).